
Cardiac Marker Market Size Projected to Reach USD 28.27 billion by 2031 at a 13.2% CAGR | The Insight Partners
The cardiac marker market analysis focuses on high-sensitivity assays (hs-Cardiac Troponin) and multiplex biomarker panels. These advancements are expected to accelerate the market growth in the coming years. The report runs an in-depth analysis of market trends, key players, and future opportunities.
/EIN News/ -- US & Canada, May 28, 2025 (GLOBE NEWSWIRE) -- According to a new comprehensive report from The Insight Partners, the cardiac marker market is witnessing significant growth owing to the rising prevalence of Cardiovascular diseases (CVDs) and growing technological advancements in diagnostic tools.
The global cardiac marker testing market is projected to grow is driven by the rising prevalence of cardiovascular diseases, an aging population, and the demand for early and accurate diagnostics. Key players include Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Danaher Corporation (Beckman Coulter), Thermo Fisher Scientific, bioMérieux, Becton, Dickinson and Company, and Bio-Rad Laboratories. Innovations such as high-sensitivity troponin assays and AI-integrated diagnostics are enhancing detection accuracy and patient outcomes.
To explore the valuable insights in the Cardiac Marker Market report, you can easily download a sample PDF of the report – https://www.theinsightpartners.com/sample/TIPRE00037802/
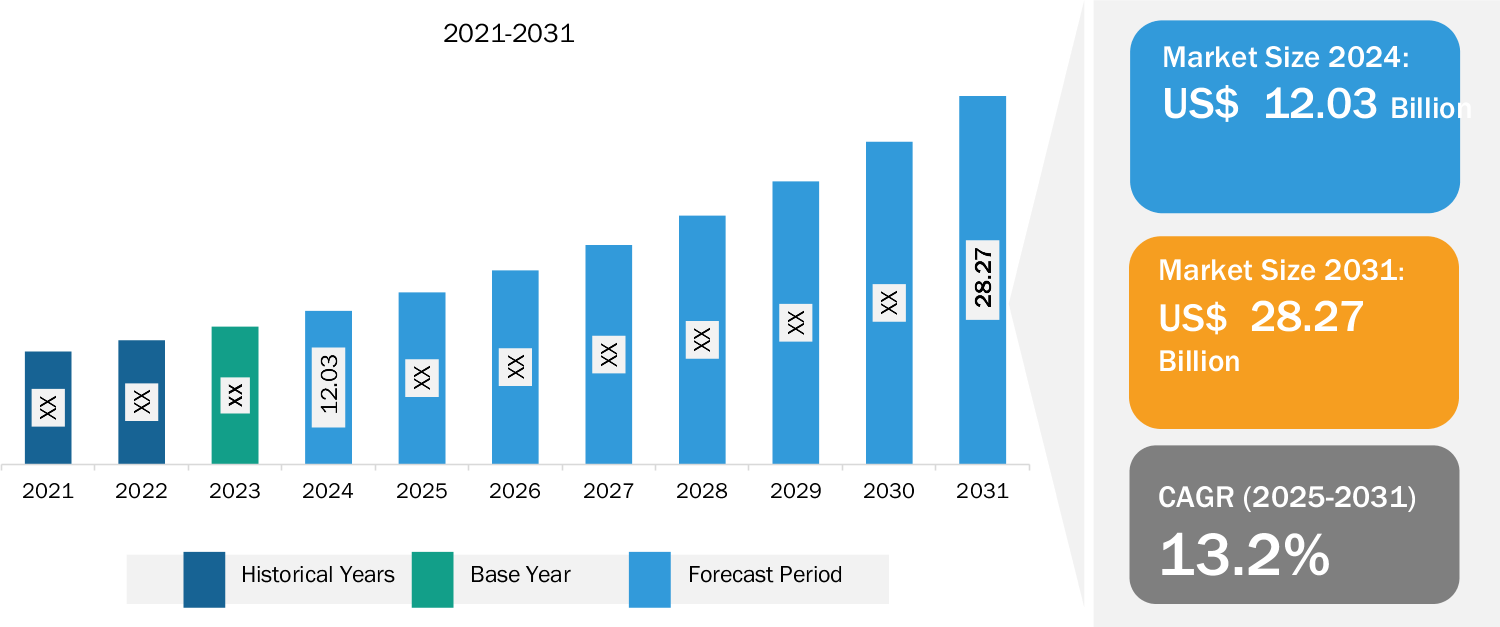
Overview of Report Findings
1. Market Growth: The cardiac marker market value is expected to reach US$ 28.27 billion by 2031 from US$ 12.03 billion in 2024; the market is anticipated to register a CAGR of 13.2% during 2025–2031. The market growth is attributed to the rising prevalence of CVDs, increasing technological advancements in diagnostic tools, and growing demand for point-of-care testing.
2. Rising Prevalence of CVDs: The increasing incidence of cardiovascular diseases (CVDs) is driving the growth of the cardiac marker market. CVDs, including heart attacks, heart failure, and stroke, represent the leading cause of mortality globally, resulting in over 17 million deaths annually, as reported by the World Health Organization. A sedentary lifestyle, poor diet, tobacco smoking, obesity, diabetes, and hypertension are some of the leading causes of the increased burden of CVDs, especially among aging populations and developing countries.
The rise in disease prevalence of CVDs increases the adoption of early and precise diagnostic devices to identify heart-associated complications. Cardiac biomarkers such as troponin, creatine kinase-MB (CK-MB), and B-type natriuretic peptide (BNP) are essential for the diagnosis of acute coronary syndromes, heart failure, and other cardiac ailments. As more patients are under threat, hospitals and medical professionals are relying on these tests to enhance clinical decision-making and outcomes.
The focus on early diagnosis and early treatment to avoid complications or minimize mortality is further enhancing the uptake of cardiac marker testing. Since CVDs are anticipated to be a heavy economic and social burden on healthcare systems, the demand for accurate, fast, and high-sensitivity cardiac biomarker assays will continue to rise steadily in the coming years.
3. Technological Advancements in Diagnostic Tools:
Advancements in diagnostic equipment are propelling the cardiac marker market growth by facilitating a speedier, more precise, and affordable detection of cardiovascular diseases (CVDs). Moreover, the advancements in assay sensitivity, automation, and data analysis have reshaped the application of cardiac biomarkers within clinical environments. The use of high-sensitivity assays, particularly for cardiac troponins, facilitates the detection of myocardial infarction at significantly earlier levels, typically one to three hours after symptoms develop. Such preclinical detection plays a critical role in enhancing patient outcomes and starting early interventions.
The evolution of point-of-care (POC) testing devices has further revolutionized cardiac diagnostics. These handheld, easy-to-use devices offer quick results at the bedside or in the emergency care environment. POC testing is used in ambulances, rural clinics, and distant communities where laboratory facilities are limited.
Automation and integration with electronic health platforms also increase the value of cardiac marker testing. Modern diagnosis systems use AI and machine learning algorithms to analyze biomarker levels alongside patient information, making more precise risk stratification and more tailored treatment plans.
Also, multiplex biomarker platforms allow for the parallel analysis of multiple cardiac markers to provide a complete picture of a patient's cardiac status through a single test. Diagnostic uncertainty is minimized, and clinical decision-making is enhanced.
These advancements enhance the precision and rapidity of cardiac diagnostics, as well as enhance the accessibility of testing. With healthcare systems placing more emphasis on early intervention and customized care, these advancements are poised to fuel long-term cardiac marker market growth during the forecast period.
4. Geographical Insights: In 2024, North America led the market with a substantial revenue share, followed by Europe and Asia Pacific. Asia Pacific is expected to register the highest CAGR during the forecast period.
Market Segmentation
- Based on type of marker, the cardiac marker market is segmented into troponin, B-type natriuretic peptide, creatine kinase MB, myoglobin, and others. The troponin segment held the largest market share in 2024.
- By indication, the cardiac marker market is divided into congestive heart failure, myocardial infarction, acute coronary syndrome, and others. The acute coronary syndrome segment dominated the market in 2024.
- In terms of end user, the cardiac marker market is categorized into hospitals, diagnostic laboratories, point-of-care testing facilities, and others. The hospitals segment dominated the market in 2024.
- The cardiac marker market is segmented into five major regions: North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America.
Stay Updated on The Latest Cardiac Marker Market Trends: https://www.theinsightpartners.com/sample/TIPRE00037802/
Competitive Strategy and Development
- Key players: Abbott Diagnostics, Roche Diagnostics, bioMérieux SA, Thermo Fisher Scientific Inc., Beckman Coulter, Inc. (Danaher); Bio-Rad Laboratories; Trinity Biotech Plc; Creative Diagnostics; Diazyme Laboratories, Inc; Siemens AG, and Quidel Corporation are among the major companies operating in the cardiac marker market.
- Trending topics: High-Sensitivity Cardiac Biomarkers, Artificial Intelligence and Big Data Integration, Multiplex Biomarker Panels, Wearable and Remote Monitoring Technologies, etc.
Headlines on Cardiac Marker
- Global medical devices and solutions provider Mindray has announced the introduction and global launch of two new high-sensitivity troponin I (hs-cTnI) and NT-proBNP cardiac biomarkers. The additions enhance Mindray's diverse portfolio of cardiac biomarkers for diagnosing and managing cardiovascular diseases (CVDs), according to a written statement issued recently on the launch.
- Pathkind Labs, in collaboration with Roche Diagnostics, is all set to release the cardiac biomarker- NTProBNP to detect heart failure in patients and will be used in the management of Type 2 Diabetes (T2DM).
- FDA Clears Cardiac Troponin I Test for Myocardial Infarction Diagnosis.
Purchase Premium Copy of Global Cardiac Marker Market Size and Growth Report (2020-2030) at: https://www.theinsightpartners.com/buy/TIPRE00037802/
Conclusion
The cardiac marker market is poised for sustained growth, driven by the increasing prevalence of cardiovascular diseases as well as the growing demand for early and accurate diagnostics. Advancements in high-sensitivity assays, point-of-care testing, and multiplex platforms have enhanced clinical decision-making and improved patient outcomes. The integration of artificial intelligence and personalized medicine is elevating the role of biomarkers in risk assessment and treatment monitoring. With aging populations and rising health awareness, cardiac marker testing is expanding across hospitals, emergency settings, and home care. As healthcare shifts toward prevention and precision, cardiac biomarkers are becoming indispensable tools, ensuring timely intervention and improved cardiovascular care worldwide. This positions the market for continued innovation and global expansion in the coming years.
The report from The Insight Partners provides several stakeholders—including manufacturers of troponin, B-type natriuretic peptide, creatine kinase MB, and myoglobin—with valuable insights to successfully navigate this evolving market landscape and unlock new opportunities.
Talk to Us Directly: https://tawk.to/chat/5d5a708ceb1a6b0be6083008/1i44d98rb
Trending Related Reports:
https://www.theinsightpartners.com/en/reports/cardiac-marker-analyzer-market
https://www.theinsightpartners.com/en/reports/cardiac-safety-service-market
https://www.theinsightpartners.com/en/reports/cardiac-poc-testing-devices-market
https://www.theinsightpartners.com/en/reports/cardiac-arrhythmia-therapeutics-market
About Us:
The Insight Partners is a one stop industry research provider of actionable intelligence. We help our clients in getting solutions to their research requirements through our syndicated and consulting research services. We specialize in industries such as Semiconductor and Electronics, Aerospace and Defense, Automotive and Transportation, Biotechnology, Healthcare IT, Manufacturing and Construction, Medical Device, Technology, Media and Telecommunications, Chemicals and Materials.
Contact Us:
If you have any queries about this report or if you would like further information, please contact us:
Contact Person: Ankit Mathur
E-mail: ankit.mathur@theinsightpartners.com
Phone: +1-646-491-9876

Distribution channels: Banking, Finance & Investment Industry, Business & Economy, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release


